According to the NIH, the cornerstones of science advancement are rigor in designing and performing scientific research and the reproducibility of biomedical research findings.’
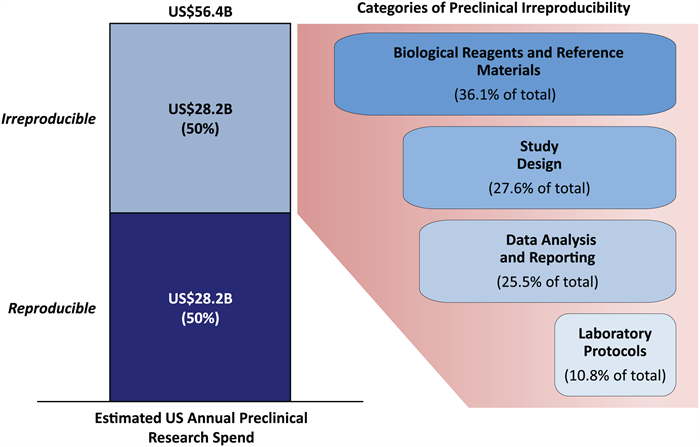
A 2015 study by the journal Nature found that poor materials made the largest contribution to reproducibility problems, at 36%, followed by study design at 28% and data analysis at 26%. The team estimates the overall rate of irreproducibility at 53%, but cautions that the true rate could be anywhere between 18% and 89%. That puts the potential economic cost of irreproducibility anywhere from $10 billion to $50 billion per year.
HOW DOES WICELL ADDRESS THIS ISSUE IN THE SCIENTIFIC COMMUNITY?
WiCell offers cell lines with release testing that follows best practice in the research process.
WiCell offers best-practice services that will help you and your lab ensure that your time and research dollars are not wasted!
WiCell’s Short Tandem Repeat (STR) and Karyotype tests can be used to address the NIH’s new grant application guidance regarding the authentication of key biological resources.
Key take-aways from NIH’s blog post on “Scientific Rigor” published January 29, 2016, by the NIH Deputy Director for Extramural Research include:
“Key cell lines might be authenticated by chromosomal analysis or short tandem repeat (STR) profiling.”
“Include a plan to independently verify the identity and activity of the product before use.”
“If the product will be used long-term, consider the stability of the product and how the validity of the product will be assessed over time.”
Review WiCell’s Sample Submission Requirements and Instructions.